
Restoring Healthy Brain Function
Depression is caused by identifiable changes in brain networks. SAINT therapy restores neural networks, allowing individuals to feel more like themselves.
Restoring Healthy Brain Function
Depression is caused by identifiable changes in brain networks. SAINT therapy restores neural networks, allowing individuals to feel more like themselves.
Precision treatment for severe depression
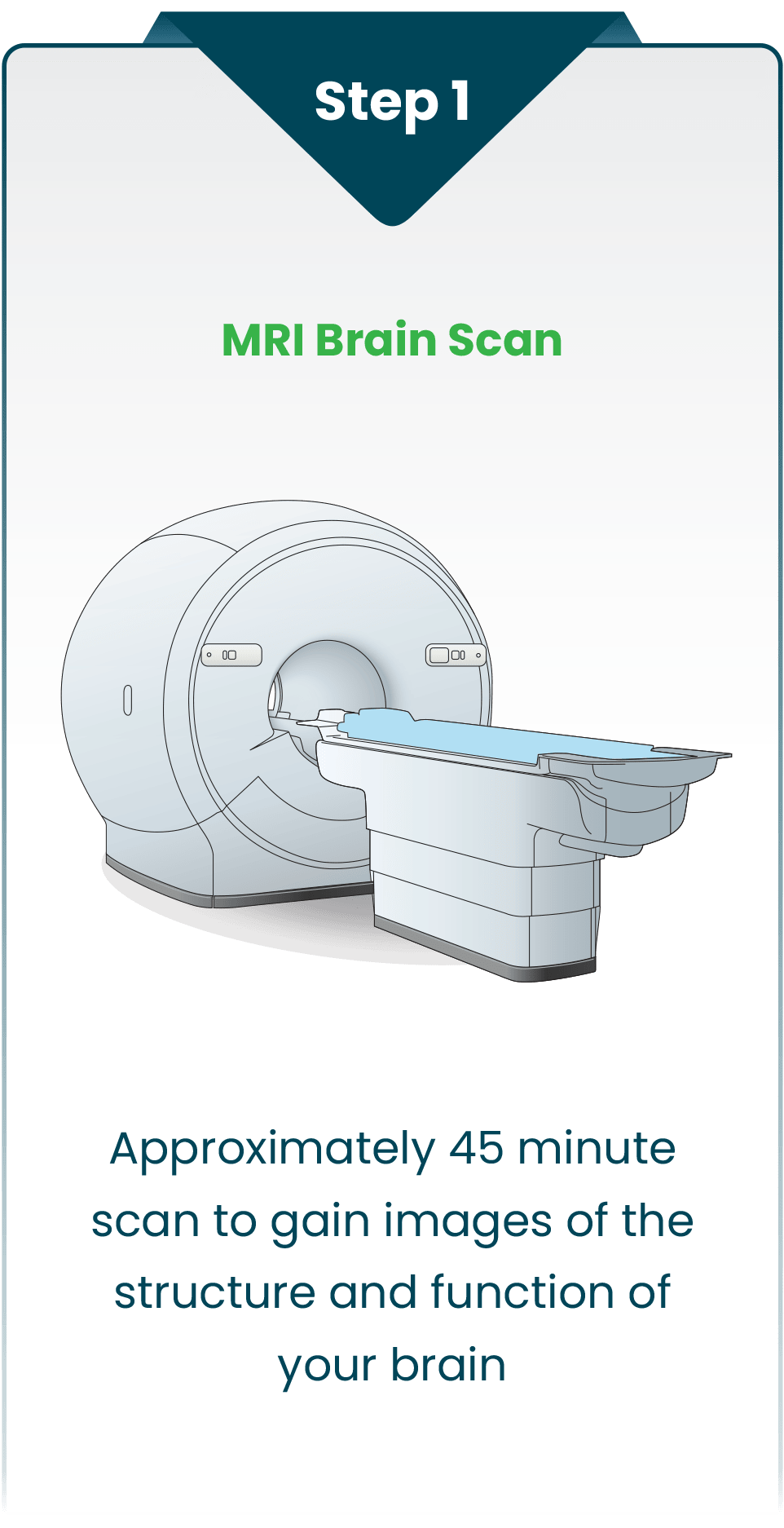
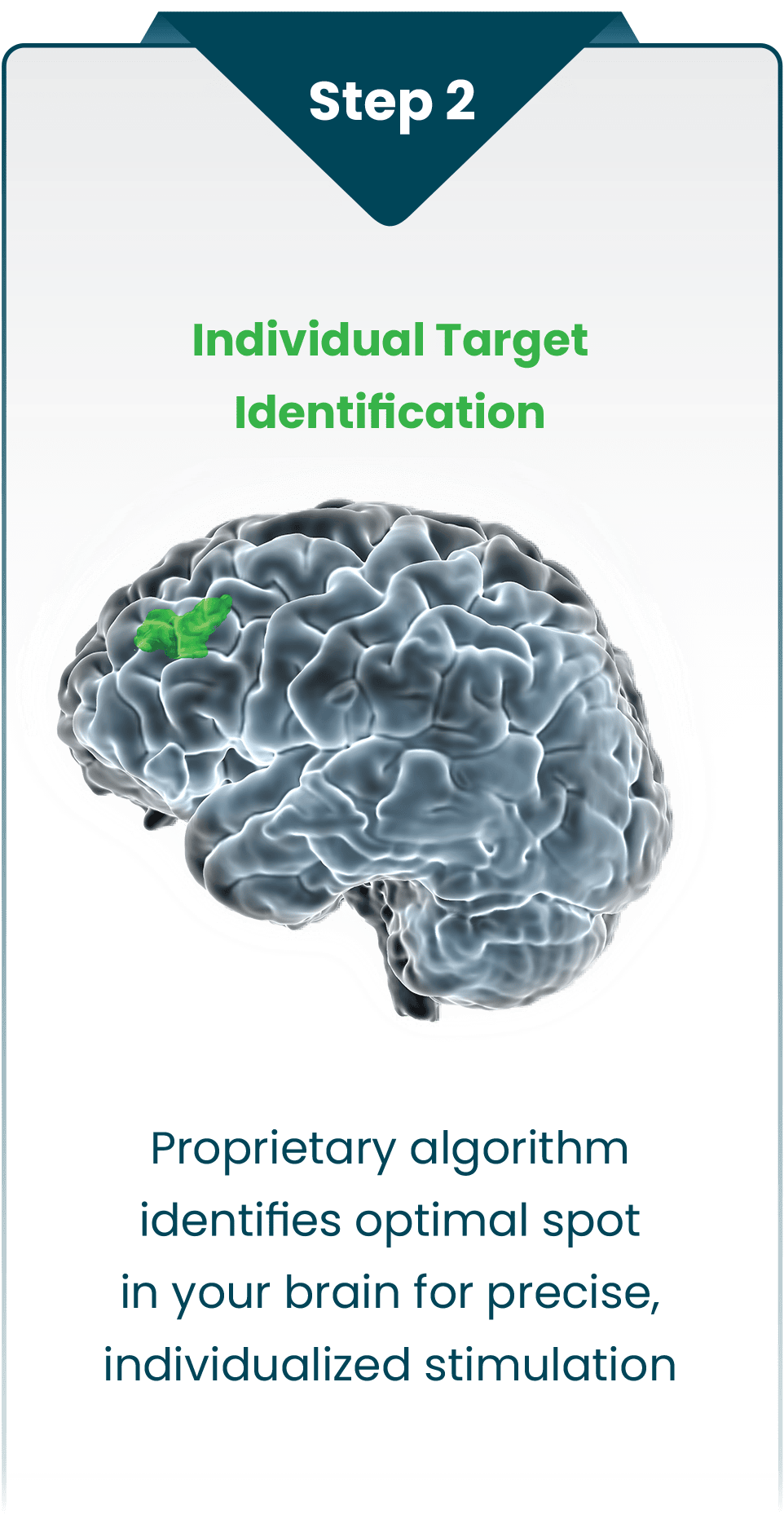
The SAINT neuromodulation system identifies the optimal therapy target in each person’s brain by using breakthrough algorithms with structural and functional MRI for precise, personalized treatment.
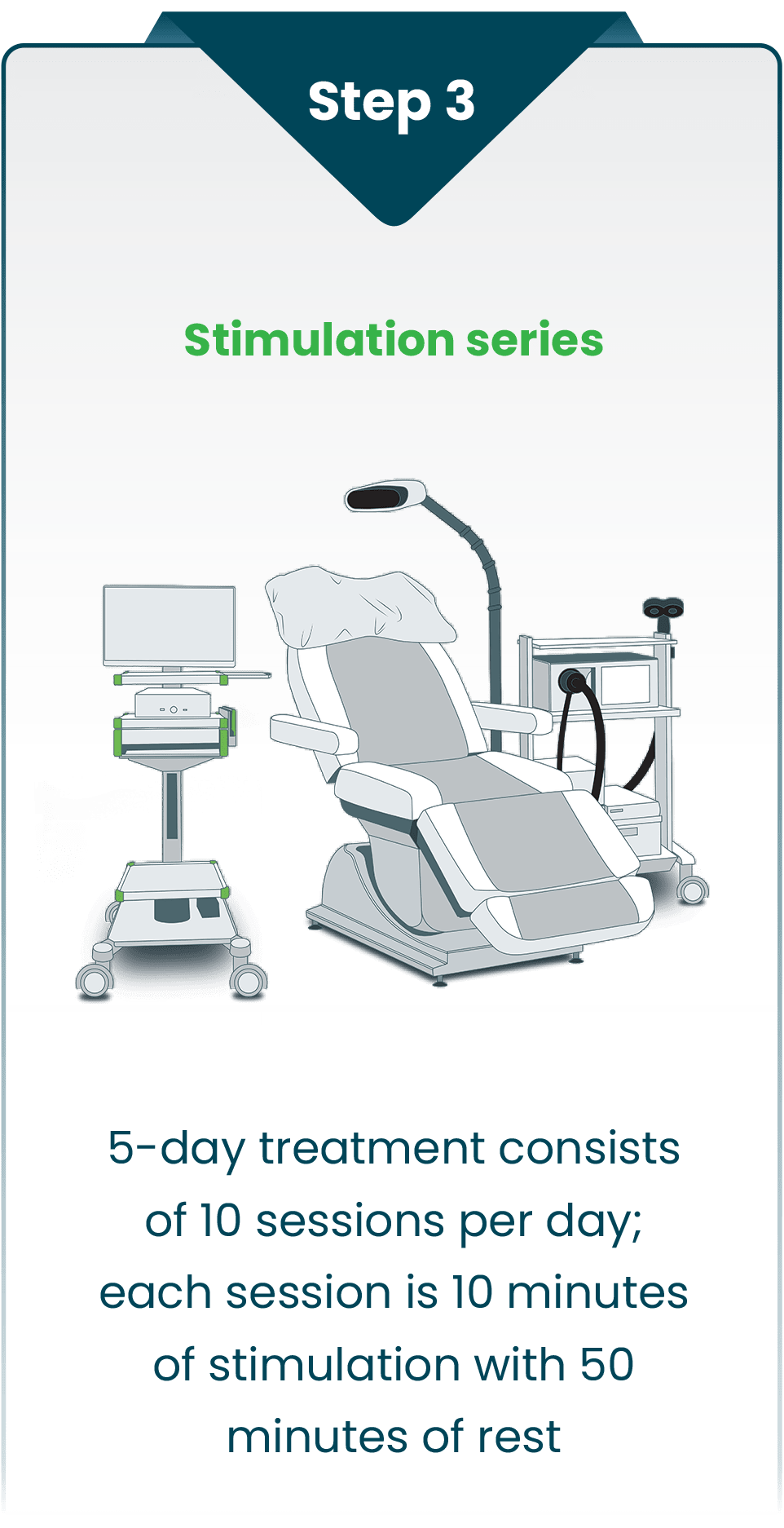
By precisely stimulating a person’s identified target with a specialized, non-invasive pattern of repetitive magnetic pulses, SAINT effectively modifies activity in brain networks related to major depression and restores mood balance.
Magnus Medical Announces Commercial Launch of Groundbreaking
SAINT Neuromodulation System
First customer sites include the University of Arkansas for Medical Sciences, MUSC Health, and Calif.-based Acacia Clinics and Kaizen Brain Center. Read the Commercial Launch Press Release
Brain stimulation poised to move from last resort to
frontline treatment
Researchers hope to hone noninvasive approaches to better treat depression and other neurological conditions. Read the PNAS article here.

Patrick Kennedy - “A Moonshot For Inner Space”
Patrick J. Kennedy writes about a watershed moment brought about by neuroscientists to gain ground against diseases of the brain. Read the article.

Neuromodulation and
Mental
Health
Magnus Medical is creating the next generation of neuromodulation technology to improve quality of life rapidly and effectively.

Work With Us
Our team is growing. We are looking for individuals who are personally and boldly committed to improving treatment for mental health. Please view our open positions here .